A Review on Lipases: Sources, Assays, Immobilization Techniques on Nanomaterials and Applications

Lipase is a highly utilized enzyme that holds significant value in various biotechnological and industrial applications, such as the food, paper, and oleochemical sectors, as well as in pharmaceutical contexts. Nevertheless, the application of the substance is relatively challenging and costly due to its aqueous solubility and instability. The immobilization technique is frequently employed to enhance the enzymatic activity and stability of lipase, and this approach has demonstrated considerable potential. The performance of immobilized lipase on nanomaterials (NMs) is subject to various factors, including the immobilization mechanisms and the specific type of matrix employed. This review examines recent advancements, mechanisms, and effects of nanomaterials (NMs) on lipase immobilization and activity. We also discuss different methods for lipase activity determination. The potential for multiple applications of immobilized lipases has been taken into consideration.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve
Similar content being viewed by others
Lipases: sources, immobilization methods, and industrial applications
Article 02 August 2019
Industrial applications of immobilized nano-biocatalysts
Article 01 October 2021

Overview of Immobilized Enzymes’ Applications in Pharmaceutical, Chemical, and Food Industry
Chapter © 2020
References
- Houde, A., et al. (2004). Lipases and their industrial applications: an overview. Applied biochemistry and biotechnology,118, 155–170.
- Remonatto, D., et al. (2022). Applications of immobilized lipases in enzymatic reactors: A review. Process Biochemistry,114, 1–20. Google Scholar
- Anobom, C. D., et al. (2014). From structure to catalysis: recent developments in the biotechnological applications of lipases. BioMed Research International, 2014, 684506
- Fotiadou, R., et al. (2021). Green synthesized magnetic nanoparticles as effective nanosupport for the immobilization of lipase: Application for the synthesis of lipophenols. Nanomaterials, 11(2), 458.
- Florindo, C., et al. (2019). Deep eutectic solvents: Overcoming 21st century challenges. Current Opinion in Green and Sustainable Chemistry,18, 31–36. Google Scholar
- Sangeetha, R., Arulpandi, I., & Geetha, A. (2011). Bacterial lipases as potential industrial biocatalysts: An overview. Research Journal of Microbiology,6(1), 1. Google Scholar
- Sharma, A., et al. (2021). Employment of polysaccharides in enzyme immobilization. Reactive and Functional Polymers,167, 105005. Google Scholar
- Sankaran, R., Show, P. L., & Chang, J. S. (2016). Biodiesel production using immobilized lipase: Feasibility and challenges. Biofuels, Bioproducts and Biorefining,10(6), 896–916. Google Scholar
- Cavalcante, F. T. T., et al. (2021). Opportunities for improving biodiesel production via lipase catalysis. Fuel,288, 119577. Google Scholar
- Cavalcante, F. T. T., et al. (2021). Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts,11(10), 1222. MathSciNetGoogle Scholar
- Filho, D. G., Silva, A. G., & Guidini, C. Z. (2019). Lipases: Sources, immobilization methods, and industrial applications. Applied Microbiology and Biotechnology,103, 7399–7423. Google Scholar
- Smoum, R., et al. (2012). Boron containing compounds as protease inhibitors. Chemical Reviews,112(7), 4156–4220. Google Scholar
- Tyndall, J. D. A., et al. (2002). Crystal structure of a thermostable lipase from Bacillus stearothermophilus P1. Journal of Molecular Biology,323(5), 859–869. Google Scholar
- Nitbani, F. O., et al. (2020). Preparation of fatty acid and monoglyceride from vegetable oil. Journal of Oleo Science, 69(4), 277–295.
- Navvabi, A., et al. (2018). Novel lipases discovery specifically from marine organisms for industrial production and practical applications. Process biochemistry, 70, 61–70.
- Tavares, F., et al. (2018). Use of castor bean seeds as lipase source for hydrolysis of crambe oil. Industrial Crops and Products,124, 254–264. Google Scholar
- Avelar, M. H. M., et al. (2013). Hydrolysis of vegetable oils catalyzed by lipase extract powder from dormant castor bean seeds. Industrial Crops and Products,44, 452–458. Google Scholar
- Okino-Delgado, C. H., & Fleuri, L. F. (2014). Obtaining lipases from byproducts of orange juice processing. Food Chemistry,163, 103–107. Google Scholar
- Chen, C.-C., et al. (2019). Two novel lipases purified from rice bran displaying lipolytic and esterification activities. International Journal of Biological Macromolecules,139, 298–306. Google Scholar
- Amid, M., et al. (2015). A novel aqueous two phase system composed of surfactant and xylitol for the purification of lipase from pumpkin (Cucurbita moschata) seed sand recycling of phase components. Molecules,20(6), 11184–11201. Google Scholar
- Zarai, Z., et al. (2012). Purification and biochemical properties of Hexaplex trunculus digestive lipase. Process Biochemistry,47(12), 2434–2439. Google Scholar
- Mendes, A. A., et al. (2010). Anaerobic biodegradability of dairy wastewater pretreated with porcine pancreas lipase. Brazilian Archives of Biology and Technology,53, 1279–1284. Google Scholar
- Liaquat, M., & Apenten, R. K. O. (2000). Synthesis of low molecular weight flavor esters using plant seedling lipases in organic media. Journal of Food Science,65(2), 295–299. Google Scholar
- Tuter, M., et al. (2003). Partial purification of Nigella sativa L. seed lipase and its application in transesterification reactions. Journal of the American Oil Chemists’ Society,80(1), 43–48. Google Scholar
- Xia, X., et al. (2009). Wheat germ lipase catalyzed kinetic resolution of secondary alcohols in non-aqueous media. Biotechnology Letters,31, 83–87. Google Scholar
- King, J. W., et al. (2001). Supercritical fluid extraction of Vernonia galamensis seeds. Industrial Crops and Products,14(3), 241–249. Google Scholar
- Sahoo, R. K., Sahu, A., & Subudhi, E. (2020). Bioremediation of hydrocarbon using bacterial lipase from waste biomass. Iranian Journal of Science and Technology, Transactions A: Science,44, 1287–1293. Google Scholar
- Abou Taleb, M., et al. (2022). Bioscouring of wool fibres using immobilized thermophilic lipase. International Journal of Biological Macromolecules,194, 800–810. Google Scholar
- Zehani, N., et al. (2015). A microconductometric biosensor based on lipase extracted from Candida rugosa for direct and rapid detection of organophosphate pesticides. International Journal of Environmental Analytical Chemistry,95(5), 466–479. Google Scholar
- Hasanah, U., et al. (2019). Construction of a hydrogel pectin-based triglyceride optical biosensor with immobilized lipase enzymes. Biosensors,9(4), 135. Google Scholar
- Ktata, A., et al. (2020). Purification, biochemical and molecular study of lipase producing from a newly thermoalkaliphilic Aeribacillus pallidus for oily wastewater treatment. The Journal of Biochemistry,167(1), 89–99. Google Scholar
- Saraswat, R., et al. (2017). Evaluation of alkali and thermotolerant lipase from an indigenous isolated Bacillus strain for detergent formulation. Electronic Journal of Biotechnology,30, 33–38. Google Scholar
- Liu, W., Li, M., & Yan, Y. (2017). Heterologous expression and characterization of a new lipase from Pseudomonas fluorescens Pf0–1 and used for biodiesel production. Scientific Reports,7(1), 15711. Google Scholar
- Memarpoor-Yazdi, M., Karbalaei-Heidari, H. R., & Khajeh, K. (2017). Production of the renewable extremophile lipase: Valuable biocatalyst with potential usage in food industry. Food and Bioproducts Processing,102, 153–166. Google Scholar
- Sikora, A., et al. (2017). Enantioseparation of (RS)-atenolol with the use of lipases immobilized onto new-synthesized magnetic nanoparticles. Tetrahedron: Asymmetry,28(2), 374–380. MathSciNetGoogle Scholar
- Shalini, P., et al. (2023). Synthesis and characterisation of lipase immobilised magnetic nanoparticles and its role as a catalyst in biodiesel production. Materials Today: Proceedings,80, 2725–2730. Google Scholar
- Ding, L.-N., et al. (2019). Advances in plant GDSL lipases: From sequences to functional mechanisms. Acta Physiologiae Plantarum,41, 1–11. Google Scholar
- Sundaramahalingam, M. A., et al. (2021). An encapsulated report on enzyme-assisted transesterification with an allusion to lipase. 3 Biotech,11, 1–31. Google Scholar
- Santos, K. C., et al. (2013). Characterization of the catalytic properties of lipases from plant seeds for the production of concentrated fatty acids from different vegetable oils. Industrial Crops and Products,49, 462–470. Google Scholar
- Copeland, L. O., & Mcdonald, M. F. (2012). Principles of seed science and technology. Springer Science & Business Media. Google Scholar
- Salihu, A., et al. (2012). Lipase production: An insight in the utilization of renewable agricultural residues. Resources, Conservation and Recycling,58, 36–44. Google Scholar
- Murphy, D. J. (2001). The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in Lipid Research,40(5), 325–438. Google Scholar
- Quiroga, A. D., & Lehner, R. (2012). Liver triacylglycerol lipases. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids,1821(5), 762–769. Google Scholar
- Kurtovic, I., et al. (2009). Lipases from mammals and fishes. Reviews in Fisheries Science,17(1), 18–40. Google Scholar
- Sarmah, N., et al. (2018). Recent advances on sources and industrial applications of lipases. Biotechnology Progress,34(1), 5–28. Google Scholar
- Malter, K. E. (2022). Investigating the role of contractile injection system effectors in host-microbe interactions. San Diego State University. Google Scholar
- Harrison, J. F., Woods, H. A., & Roberts, S. P. (2012). Ecological and environmental physiology of insects. OUP Oxford. Google Scholar
- Verma, N., Thakur, S., & Bhatt, A. K. (2012). Microbial lipases: Industrial applications and properties (a review). International Research Journal of Biological Sciences,1(8), 88–92. Google Scholar
- Borrelli, G. M., & Trono, D. (2015). Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. International Journal of Molecular Sciences,16(9), 20774–20840. Google Scholar
- Fatima, S., et al. (2021). Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnology and Applied Biochemistry,68(3), 445–458. Google Scholar
- Mahadik, N. D., et al. (2002). Production of acidic lipase by Aspergillus niger in solid state fermentation. Process Biochemistry,38(5), 715–721. Google Scholar
- Long, Z.-D., Xu, J.-H., & Pan, J. (2007). Significant improvement of Serratia marcescens lipase fermentation, by optimizing medium, induction, and oxygen supply. Applied Biochemistry and Biotechnology,142, 148–157. Google Scholar
- Rawat, I., et al. (2013). Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Applied Energy,103, 444–467. Google Scholar
- Benjamin, S., & Pandey, A. (2001). Isolation and characterization of three distinct forms of lipases from Candida rugosa produced in solid state fermentation. Brazilian Archives of Biology and Technology,44, 213–221. Google Scholar
- Mehta, A., Bodh, U., & Gupta, R. (2017). Fungal lipases: A review. Journal of Biotech Research,8, 58. Google Scholar
- Kumar, A., & Kanwar, S. S. (2012). Lipase production in solid-state fermentation (SSF): Recent developments and biotechnological applications. Dynamic Biochemistry, Process Biotechnology and Molecular Biology,6(1), 13–27. Google Scholar
- Ruchi, G., Anshu, G., & Khare, S. K. (2008). Lipase from solvent tolerant Pseudomonas aeruginosa strain: Production optimization by response surface methodology and application. Bioresource Technology,99(11), 4796–4802. Google Scholar
- Hasan, F., Shah, A. A., & Hameed, A. (2009). Methods for detection and characterization of lipases: A comprehensive review. Biotechnology Advances,27(6), 782–798. Google Scholar
- Gupta, R., et al. (2003). Lipase assays for conventional and molecular screening: An overview. Biotechnology and Applied Biochemistry,37(1), 63–71. Google Scholar
- Soberón-Chávez, G., & Palmeros B. (1994). Pseudomonas lipases: molecular genetics and potential industrial applications. Critical reviews in Microbiology, 20(2), 95–105.
- Coradi, G. V., et al. (2013). Comparing submerged and solid-state fermentation of agro-industrial residues for the production and characterization of lipase by Trichoderma harzianum. Annals of Microbiology,63(2), 533–540. Google Scholar
- Zottig, X., Meddeb-Mouelhi, F., & Beauregard, M. (2016). Development of a high-throughput liquid state assay for lipase activity using natural substrates and rhodamine B. Analytical Biochemistry,496, 25–29. Google Scholar
- Chandra, P., Singh, R., & Arora, P. K. (2020). Microbial lipases and their industrial applications: A comprehensive review. Microbial Cell Factories,19(1), 1–42. Google Scholar
- Jaeger, K.-E., & Kovacic, F. (2014). Determination of lipolytic enzyme activities. Pseudomonas methods and protocols (pp. 111–134). Springer. Google Scholar
- Kolakowski, E. (2005). Analysis of proteins, peptides, and amino acids in foods. Methods of analysis of food components and additives, 60–93
- Lanka, S., & Latha, J. N. L. (2015). A short review on various screening methods to isolate potential lipase producers: Lipases-the present and future enzymes of biotech industry. International Journal of Biological Chemistry,9(5), 207–219. Google Scholar
- Bou, R., et al. (2008). Determination of hydroperoxides in foods and biological samples by the ferrous oxidation–xylenol orange method: A review of the factors that influence the method’s performance. Analytical Biochemistry,377(1), 1–15. Google Scholar
- Gupta, R., et al. (2003). Lipase assays for conventional and molecular screening: an overview. Biotechnology and Applied Biochemistry, 37(1), 63–71.
- Lee, L.-C., et al. (2011). Characterization of codon-optimized recombinant Candida rugosa lipase 5 (LIP5). Journal of Agricultural and Food Chemistry,59(19), 10693–10698. Google Scholar
- Tymecki, Ł., et al. (2009). UV-PEDD photometry dedicated for bioanalytical uses. Analyst, 134(7), 1333–1337.
- Stoytcheva, M., et al. (2012). Analytical methods for lipases activity determination: A review. Current Analytical Chemistry,8(3), 400–407. Google Scholar
- Thomsen, R., et al. (2015). Synthetic cannabimimetic agents metabolized by carboxylesterases. Drug Testing and Analysis,7(7), 565–576. Google Scholar
- Cruz, M., et al. (2019). Monitoring enzymatic hydroesterification of low-cost feedstocks by Fourier transform infrared spectroscopy. Catalysts,9(6), 535. Google Scholar
- Lundin, M. D., et al. (2015). Liquid CO2 as a safe and benign solvent for the ozonolysis of fatty acid methyl esters. ACS Sustainable Chemistry & Engineering,3(12), 3307–3314. Google Scholar
- Lam, H. (2003). Surface free fatty acids and hydroperoxides production and measurement on milled rice. London: University of Arkansas. Google Scholar
- Bell, C. W., et al. (2013). High-throughput fluorometric measurement of potential soil extracellular enzyme activities. JoVE (Journal of Visualized Experiments),81, e50961. Google Scholar
- Angelin, J., & KavithaM., (2022). Extremophilic fungal lipases: screening, purification, assay, and applications. Extremophilic Fungi, pp. 395–438.
- Kitchener, B. G. B., Wainwright, J., & Parsons, A. J. (2017). A review of the principles of turbidity measurement. Progress in Physical Geography,41(5), 620–642. Google Scholar
- Hu, Q., et al. (2021). In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocolloids,112, 106340. Google Scholar
- Seefelder, W., et al. (2008). Esters of 3-chloro-1, 2-propanediol (3-MCPD) in vegetable oils: Significance in the formation of 3-MCPD. Food Additives and Contaminants,25(4), 391–400. Google Scholar
- Ishiyama, N., et al. (2017). Lipoprotein lipase does not increase significantly in the postprandial plasma. Clinica Chimica Acta,464, 204–210. Google Scholar
- Jones, A., et al. (2019). Multiplexed immunosensors and immunoarrays. Analytical Chemistry,92(1), 345–362. Google Scholar
- Gupta, R., Gupta, N., & Rathi, P. (2004). Bacterial lipases: An overview of production, purification and biochemical properties. Applied Microbiology and Biotechnology,64(6), 763–781. Google Scholar
- Dhingra, D., Chopra, S., & Rai, D. R. (2012). Stabilization of raw rice bran using ohmic heating. Agricultural Research,1(4), 392–398. Google Scholar
- Elsamadony, M., et al. (2021). Advances towards understanding long chain fatty acids-induced inhibition and overcoming strategies for efficient anaerobic digestion process. Water Research,190, 116732.
- Reyes, A. L., et al. (2019). Conductometric method for rapid lipase activity quantification. International Journal of Electrochemical Science,14, 10508–10521. Google Scholar
- Popkova, Y., et al. (2020). Differences in the lipid patterns during maturation of 3T3-L1 adipocytes investigated by thin-layer chromatography, gas chromatography, and mass spectrometric approaches. Analytical and Bioanalytical Chemistry,412(10), 2237–2249. Google Scholar
- Dołowy, M., & Pyka A., (2015). Chromatographic methods in the separation of long-chain mono- and polyunsaturated fatty acids. Journal of Chemistry, 2015, 120830.
- Al HamouiDitBanni, G., et al. (2021). Investigation of lipase-ligand interactions in porcine pancreatic extracts by microscale thermophoresis. Analytical and Bioanalytical Chemistry,413(14), 3667–3681. Google Scholar
- Ray, A. (2012). Application of lipase in industry. Asian Journal of Pharmacy and Technology,2(2), 33–37. Google Scholar
- Vakhlu, J. (2006). Yeast lipases: Enzyme purification, biochemical properties and gene cloning. Electronic Journal of Biotechnology,9(1), 0–0. Google Scholar
- Sharma, S., & Kanwar, S. S. (2014). Organic solvent tolerant lipases and applications. The Scientific World Journal, 2014, 625258.
- Gurung, N., et al. (2013). A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. BioMed research international, 2013, 329121.
- Yetisen, A. K., et al. (2016). Nanotechnology in textiles. ACS Nano,10(3), 3042–3068. Google Scholar
- Holme, I. (2007). Innovative technologies for high performance textiles. Coloration Technology,123(2), 59–73. Google Scholar
- Joseph, P., & Tretsiakova-McNally, S. (2013). Chemical modification of natural and synthetic textile fibres to improve flame retardancy. In: Handbook of fire resistant textiles. (pp. 37–67). Elsevier
- Joseph, B., Ramteke, P. W., & Thomas, G. (2008). Cold active microbial lipases: Some hot issues and recent developments. Biotechnology Advances,26(5), 457–470. Google Scholar
- Perfumo, A., Banat, I. M., & Marchant, R. (2018). Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends in Biotechnology,36(3), 277–289. Google Scholar
- Gunasekaran, V., & Das D. (2005). Lipase fermentation: Progress and prospects. Indian Journal of Biotechnology, 4, 437–445.
- Meshram, A., et al. (2019). Plant-derived enzymes: a treasure for food biotechnology. Enzymes in food biotechnology (pp. 483–502). Elsevier.
- Mehta, A., et al. (2021). The lipases and their applications with emphasis on food industry. Microbial biotechnology in food and health (pp. 143–164). Elsevier.
- Vandamme, E. J., & Soetaert, W. (2002). Bioflavours and fragrances via fermentation and biocatalysis. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology,77(12), 1323–1332. Google Scholar
- Bora, L., Gohain, D., & Das, R. (2013). Recent advances in production and biotechnological applications of thermostable and alkaline bacterial lipases. Journal of Chemical Technology & Biotechnology,88(11), 1959–1970. Google Scholar
- Rajendran, A., Palanisamy, A., & Thangavelu, V. (2009). Lipase catalyzed ester synthesis for food processing industries. Brazilian Archives of Biology and Technology,52, 207–219. Google Scholar
- Woodley, J. M. (2008). New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends in Biotechnology,26(6), 321–327. Google Scholar
- Dilip, C. V., Mulaje, S. S., & Mohalkar, R. Y. (2013). A review on actinomycetes and their biotechnological application. International Journal of Pharmaceutical Sciences and Research,4(5), 1730. Google Scholar
- Bhardwaj, K. K., & Gupta R. (2017). Synthesis of chirally pure enantiomers by lipase. Journal of Oleo Science, 66(10), 1073–1084.
- Jolvis Pou, K. R., et al. (2019). Industrial Processing of CTC Black Tea. Caffeinated and Cocoa Based Beverages, 2019, 131–162.
- Chen, Y. L., et al. (2010). Production, quality, and biological effects of oolong tea (Camellia sinensis). Food Reviews International,27(1), 1–15. Google Scholar
- Gandhi, K., et al. (2013). Modified Milk Fat and Its Applications in Food Products. Journal of Dairy Science and Technology, 2, 16–24.
- Haghi, A. K. (2011). Food science: Research and technology. CRC Press. Google Scholar
- Lafontan, M., & Langin, D. (2009). Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research,48(5), 275–297. Google Scholar
- Abd El-Hack, M. E., et al. (2016). Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. International Journal of Pharmacology,12(3), 232–248. Google Scholar
- Balthazar, E. J. (2002). Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology,223(3), 603–613. Google Scholar
- Vishnoi, N., et al. (2020). Microbial Lipases and Their Versatile Applications. Microbial enzymes: Roles and applications in industries, 207–230.
- Annenkov, G. A., et al. (2004). Wide range of the use of natural lipases and esterases to inhibit Mycobacterium tuberculosis. Problemy Tuberkuleza i Boleznei Legkikh,6, 52–56. Google Scholar
- Menzies, D., Pai, M., & Comstock, G. (2007). Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Annals of Internal Medicine,146(5), 340–354. Google Scholar
- Wanyonyi, W. C., & Mulaa, F. J. (2020). Alkaliphilic enzymes and their application in novel leather processing technology for next-generation tanneries. Advances in biochemical engineering/biotechnology, 172, 195–220.
- Moujehed, E., et al. (2022). Cleaner degreasing of sheepskins by the Yarrowia lipolytica LIP2 lipase as a chemical-free alternative in the leather industry. Colloids and Surfaces B: Biointerfaces,211, 112292. Google Scholar
- Habenicht, G. (2008). Applied adhesive bonding: A practical guide for flawless results. John Wiley & Sons. Google Scholar
- El-Khawaga, A. M., et al. (2023). Promising photocatalytic and antimicrobial activity of novel capsaicin coated cobalt ferrite nanocatalyst. Scientific Reports, 13(1), 5353.
- Bertanza, G., Collivignarelli, C., & Pedrazzani, R. (2001). The role of chemical oxidation in combined chemical-physical and biological processes: Experiences of industrial wastewater treatment. Water Science and Technology,44(5), 109–116. Google Scholar
- Cammarota, M. C., & Freire, D. M. G. (2006). A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresource Technology,97(17), 2195–2210. Google Scholar
- Veerapagu, M., et al. (2013). Screening selection identification production and optimization of bacterial lipase from oil spilled soil. Asian Journal of Pharmaceutical and Clinical Research,6(3), 62–67. Google Scholar
- Awaleh, M. O., & Soubaneh, Y. D. (2014). Waste water treatment in chemical industries: The concept and current technologies. Hydrology: Current Research,5(1), 1. Google Scholar
- Basheer, S. M., et al. (2011). Lipase from marine Aspergillus awamori BTMFW032: Production, partial purification and application in oil effluent treatment. New Biotechnology,28(6), 627–638. Google Scholar
- Sheldon, R. A. (2005). Green solvents for sustainable organic synthesis: State of the art. Green Chemistry,7(5), 267–278. Google Scholar
- Østergaard, L. H., & Olsen, H. S. (2011). Industrial applications of fungal enzymes. Industrial applications, 269–290.
- Lotti, M., et al. (2015). Effects of methanol on lipases: Molecular, kinetic and process issues in the production of biodiesel. Biotechnology Journal,10(1), 22–30. Google Scholar
- Mata, T. M., et al. (2012). Biodiesel production from corn oil via enzymatic catalysis with ethanol. Energy & Fuels,26(5), 3034–3041. Google Scholar
- Okino-Delgado, C. H., et al. (2017). Bioremediation of cooking oil waste using lipases from wastes. PLoS ONE,12(10), e0186246. Google Scholar
- Abdulmalek, S. A., & Yan Y. (2022). Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels, Bioproducts and Biorefining 16(4), 1062–1094.
- Federsel, H.-J., Moody, T. S., & Taylor, S. J. C. (2021). Recent trends in enzyme immobilization—Concepts for expanding the biocatalysis toolbox. Molecules,26(9), 2822. Google Scholar
- Aguieiras, E. C. G., Cavalcanti-Oliveira, E. D., & Freire, D. M. G. (2015). Current status and new developments of biodiesel production using fungal lipases. Fuel,159, 52–67. Google Scholar
- Ren, S., et al. (2019). Recent progress in multienzymes co-immobilization and multienzyme system applications. Chemical Engineering Journal,373, 1254–1278. Google Scholar
- Peterson, D. S., et al. (2002). Enzymatic microreactor-on-a-chip: Protein mapping using trypsin immobilized on porous polymer monoliths molded in channels of microfluidic devices. Analytical Chemistry,74(16), 4081–4088. Google Scholar
- Hwang, E. T., & Gu, M. B. (2013). Enzyme stabilization by nano/microsized hybrid materials. Engineering in Life Sciences,13(1), 49–61. Google Scholar
- Carlsson, N., et al. (2014). Enzymes immobilized in mesoporous silica: A physical–chemical perspective. Advances in colloid and interface science,205, 339–360. Google Scholar
- Tapdigov, S. Z. (2021). The bonding nature of the chemical interaction between trypsin and chitosan based carriers in immobilization process depend on entrapped method: A review. International Journal of Biological Macromolecules,183, 1676–1696. Google Scholar
- Mohamad, N. R., et al. (2015). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnology & Biotechnological Equipment,29(2), 205–220. MathSciNetGoogle Scholar
- Rizwan, M., et al. (2009). Enhanced transdermal drug delivery techniques: An extensive review of patents. Recent Patents on Drug Delivery & Formulation,3(2), 105–124. MathSciNetGoogle Scholar
- Secundo, F. (2013). Conformational changes of enzymes upon immobilisation. Chemical Society Reviews,42(15), 6250–6261. Google Scholar
- Imam, H. T., Marr, P. C., & Marr, A. C. (2021). Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chemistry,23(14), 4980–5005. Google Scholar
- Nguyen, H. H., & Kim, M. (2017). An overview of techniques in enzyme immobilization. Applied Science and Convergence Technology,26(6), 157–163. Google Scholar
- Hu, W.-S. (2017). Engineering principles in biotechnology. John Wiley & Sons. Google Scholar
- Xu, Z.-K., Huang, X.-J., & Wan, L.-S. (2009). Surface engineering of polymer membranes. Springer Science & Business Media. Google Scholar
- DiCosimo, R., et al. (2013). Industrial use of immobilized enzymes. Chemical Society Reviews,42(15), 6437–6474. Google Scholar
- Shuai, W., et al. (2017). A review on the important aspects of lipase immobilization on nanomaterials. Biotechnology and Applied Biochemistry,64(4), 496–508. Google Scholar
- Damodaran, S. (2008). Amino acids, peptides and proteins. Fennema’s food chemistry,4, 425–439.
- Calderón, M., et al. (2010). Dendritic polyglycerols for biomedical applications. Advanced Materials,22(2), 190–218. Google Scholar
- Eisenmenger, M. J., & Reyes-De-Corcuera, J. I. (2009). High pressure enhancement of enzymes: A review. Enzyme and Microbial Technology,45(5), 331–347. Google Scholar
- Lämmerhofer, M. (2010). Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. Journal of Chromatography A,1217(6), 814–856. Google Scholar
- Sheldon, R. A., & van Pelt, S. (2013). Enzyme immobilisation in biocatalysis: Why, what and how. Chemical Society Reviews,42(15), 6223–6235. Google Scholar
- Rueda, N., et al. (2016). Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. The Chemical Record,16(3), 1436–1455. Google Scholar
- Ozaltin, K., et al. (2019). Polysaccharides based microspheres for multiple encapsulations and simultaneous release of proteases. International Journal of Biological Macromolecules,132, 24–31. Google Scholar
- Jegan Roy, J., & Emilia Abraham T. (2004). Strategies in making cross-linked enzyme crystals. Chemical Reviews 104(9), 3705–3722.
- Zucca, P., Fernandez-Lafuente, R., & Sanjust, E. (2016). Agarose and its derivatives as supports for enzyme immobilization. Molecules,21(11), 1577. Google Scholar
- Vaghari, H., et al. (2016). Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnology letters,38, 223–233. Google Scholar
- Zahirinejad, S., et al. (2021). Nano-organic supports for enzyme immobilization: Scopes and perspectives. Colloids and Surfaces B: Biointerfaces,204, 111774. Google Scholar
- Kim, J. H., et al. (2022). Post-synthetic modifications in porous organic polymers for biomedical and related applications. Chemical Society Reviews,51(1), 43–56. Google Scholar
- Selvam, T., Machoke, A., & Schwieger, W. (2012). Supported ionic liquids on non-porous and porous inorganic materials—A topical review. Applied Catalysis A: General,445, 92–101. Google Scholar
- Zhu, T., Wang, J., & Ho, G. W. (2015). Self-supported yolk–shell nanocolloids towards high capacitance and excellent cycling performance. Nano Energy,18, 273–282. Google Scholar
- Sen, D., et al. (2011). Feasibility study of enzyme immobilization on polymeric membrane: A case study with enzymatically galacto-oligosaccharides production from lactose. Journal of Membrane Science,378(1–2), 471–478. Google Scholar
- Luo, J., et al. (2015). Cascade catalysis in membranes with enzyme immobilization for multi-enzymatic conversion of CO2 to methanol. New Biotechnology,32(3), 319–327. Google Scholar
- Hola, K., et al. (2015). Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnology Advances,33(6), 1162–1176. Google Scholar
- Homaei, A. A., et al. (2013). Enzyme immobilization: An update. Journal of Chemical Biology,6, 185–205. Google Scholar
- El-Khawaga, A. M., et al. (2022). Synthesis and applicability of reduced graphene oxide/porphyrin nanocomposite as photocatalyst for waste water treatment and medical applications. Scientific Reports, 12(1): 17075. Google Scholar
- Cipolatti, E. P., et al. (2014). Current status and trends in enzymatic nanoimmobilization. Journal of Molecular Catalysis B: Enzymatic,99, 56–67. Google Scholar
- Ahmad, R., & Sardar, M. (2015). Enzyme immobilization: An overview on nanoparticles as immobilization matrix. Biochemistry and Analytical Biochemistry,4(2), 1. Google Scholar
- Andrade, M. F. C., et al. (2016). Lipase immobilized on polydopamine-coated magnetite nanoparticles for biodiesel production from soybean oil. Biofuel Research Journal,3(2), 403. Google Scholar
- Singh, A., et al. (2018). Methods of enzyme immobilization and its applications in food industry. Enzymes in Food Technology: Improvements and Innovations, 103–124.
- El-Khawaga, A. M., et al. (2022). Promising antimicrobial and azo dye removal activities of citric acid-functionalized magnesium ferrite nanoparticles. Journal of Cluster Science, 33(1), 197–213.
- Aggarwal, S., Chakravarty, A., & Ikram, S. (2021). A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. International Journal of Biological Macromolecules,167, 962–986. Google Scholar
- Jamshaid, T., et al. (2016). Magnetic particles: From preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications. TrAC Trends in Analytical Chemistry,79, 344–362. Google Scholar
- Seenuvasan, M., et al. (2013). Immobilization of pectinase on co-precipitated magnetic nanoparticles for enhanced stability and activity. Research Journal of Biotechnology,8(5), 24–30. Google Scholar
- Thakur, K., et al. (2021). Nanocarriers-based immobilization of enzymes for industrial application. 3 Biotech, 11(10), 427.
- Mohamad, N. R., et al. (2015). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnology & Biotechnological Equipment 29(2), 205–220.
- El-Boubbou, K. (2018). Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine,13(8), 929–952. Google Scholar
- Khalid, N., et al. (2022). Non-magnetic and magnetically responsive support materials immobilized peroxidases for biocatalytic degradation of emerging dye pollutants—A review. International Journal of Biological Macromolecules,207, 387–401. Google Scholar
- Bilal, M., et al. (2019). Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry,5(3), 42. Google Scholar
- Butova, V. V. E., et al. (2016). Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russian Chemical Reviews,85(3), 280. Google Scholar
- Cai, G., et al. (2021). Metal–organic framework-based hierarchically porous materials: Synthesis and applications. Chemical Reviews,121(20), 12278–12326. Google Scholar
- Ibrahim, K. S. (2013). Carbon nanotubes? Properties and applications: A review. Carbon Letters,14(3), 131–144. Google Scholar
- Gupta, S., Murthy, C. N., & Prabha, C. R. (2018). Recent advances in carbon nanotube based electrochemical biosensors. International Journal of Biological Macromolecules,108, 687–703. Google Scholar
- Miletić, N., et al. (2010). Immobilization of Candida antarctica lipase B on polystyrene nanoparticles. Macromolecular Rapid Communications,31(1), 71–74. Google Scholar
- Huang, S. H., Liao, M. H., & Chen, D. H. (2003). Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnology Progress,19(3), 1095–1100. Google Scholar
- Chen, Y. Z., Ching, C. B., & Xu, R. (2009). Lipase immobilization on modified zirconia nanoparticles: Studies on the effects of modifiers. Process Biochemistry,44(11), 1245–1251. Google Scholar
- Kim, M. I., et al. (2006). Immobilization of Mucor javanicus lipase on effectively functionalized silica nanoparticles. Journal of Molecular Catalysis B: Enzymatic,39(1–4), 62–68. Google Scholar
- SreeHarsha, N., et al. (2019). Immobilization studies of Candida Antarctica lipase B on gallic acid resin-grafted magnetic iron oxide nanoparticles. International Journal of Nanomedicine, 14, 3235–3244.
- Răcuciu, M., Creangă, D. E., & Airinei, A. (2006). Citric-acid-coated magnetite nanoparticles for biological applications. The European Physical Journal E,21, 117–121. Google Scholar
- Drechsler, U., et al. (2004). Highly efficient biocatalysts via covalent immobilization of Candida rugosa lipase on ethylene glycol-modified gold–silica nanocomposites. Advanced Materials,16(3), 271–274. Google Scholar
- Cui, Y., et al. (2010). Facile synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and application for lipase immobilization. Journal of Biotechnology,150(1), 171–174. Google Scholar
- Ren, Y., et al. (2011). Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnology,11, 1–8. Google Scholar
- Mahmood, I., et al. (2008). Lipase Immobilization on oleic acid−pluronic (L-64) block copolymer coated magnetic nanoparticles, for hydrolysis at the oil/water interface. Industrial & Engineering Chemistry Research,47(17), 6379–6385. Google Scholar
- Wu, Y., et al. (2009). In situ preparation of magnetic Fe3O4-chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresource Technology,100(14), 3459–3464. Google Scholar
- Cao, C., et al. (2014). In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technology,260, 90–97. Google Scholar
- Malar, C. G., Seenuvasan, M., & Kumar, K. S. (2019). Basic study on lipase-immobilized magnetic nanoparticles. Nanotechnology for Environmental Engineering,4, 1–6. Google Scholar
- Yuce-Dursun, B., et al. (2016). Preparation and characterization of sol–gel hybrid coating films for covalent immobilization of lipase enzyme. Journal of Molecular Catalysis B: Enzymatic,127, 18–25. Google Scholar
- Otari, S. V., et al. (2019). Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian Journal of Microbiology,59, 105–108. Google Scholar
- Ali, R. M., Elkatory, M. R., & Hamad, H. A. (2020). Highly active and stable magnetically recyclable CuFe2O4 as a heterogenous catalyst for efficient conversion of waste frying oil to biodiesel. Fuel,268, 117297. Google Scholar
- Chen, Y. Z., et al. (2008). Immobilization of lipases on hydrophobilized zirconia nanoparticles: Highly enantioselective and reusable biocatalysts. Langmuir,24(16), 8877–8884. Google Scholar
- Xie, W., & Wang, J. (2012). Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass and Bioenergy,36, 373–380. Google Scholar
- Tran, D.-T., Chen, C.-L., & Chang, J.-S. (2012). Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. Journal of Biotechnology,158(3), 112–119. Google Scholar
- Padmanabhan, S. K., et al. (2009). Sol–gel synthesis and characterization of hydroxyapatite nanorods. Particuology,7(6), 466–470. Google Scholar
- Carvalho, T., et al. (2020). Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. International Journal of Biological Macromolecules,160, 889–902. Google Scholar
- Shen, Y. F., et al. (2009). Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Separation and Purification Technology,68(3), 312–319. Google Scholar
- Atiroğlu, V. (2020). Lipase immobilization on synthesized hyaluronic acid-coated magnetic nanoparticle-functionalized graphene oxide composites as new biocatalysts: Improved reusability, stability, and activity. International Journal of Biological Macromolecules,145, 456–465. Google Scholar
- Shi, Y., et al. (2016). Immobilization of lipase by adsorption onto magnetic nanoparticles in organic solvents. Journal of Nanoscience and Nanotechnology,16(1), 601–607. Google Scholar
- Fotiadou, R., et al. (2021). Green synthesized magnetic nanoparticles as effective nanosupport for the immobilization of lipase: Application for the synthesis of lipophenols. Nanomaterials,11(2), 458. Google Scholar
- El-Batal, A. I., et al. (2016). Biodiesel production by Aspergillus niger lipase immobilized on barium ferrite magnetic nanoparticles. Bioengineering,3(2), 14. Google Scholar
- Monteiro, R. R. C., et al. (2019). Immobilization of lipase A from Candida antarctica onto chitosan-coated magnetic nanoparticles. International journal of molecular sciences,20(16), 4018. Google Scholar
- Macario, A., et al. (2013). Pure silica nanoparticles for liposome/lipase system encapsulation: Application in biodiesel production. Catalysis Today,204, 148–155. Google Scholar
- Adnan, M., et al. (2018). X-shaped ZIF-8 for immobilization rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts,8(3), 96. Google Scholar
- Badoei-dalfard, A., et al. (2019). Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renewable Energy,141, 874–882. Google Scholar
- Rafiei, S., et al. (2018). Efficient biodiesel production using a lipase@ ZIF-67 nanobioreactor. Chemical Engineering Journal,334, 1233–1241. Google Scholar
- Mehde, A. A., et al. (2018). Lipase-based on starch material as a development matrix with magnetite cross-linked enzyme aggregates and its application. International Journal of Biological Macromolecules,120, 1533–1543. Google Scholar
- Rizki, K., et al. (2020). Immobilization of lipase in silica gel from rice husk ash and its activity assay to hydrolyze palm oil. BIO Web of conference,28, 03005.
- Kumar, A., & Kanwar, S. S. (2012). An innovative approach to immobilize lipase onto natural fiber and its application for the synthesis of 2-octyl ferulate in an organic medium. Current Biotechnology,1(3), 241–248. Google Scholar
- Deng, L., et al. (2019). A core–shell structured immobilized lipase and its application in high-temperature reactions. Applied Biochemistry and Biotechnology,189, 774–786. Google Scholar
- Nuraliyah, A., et al. (2018). Immobilization of Candida rugosa lipase by adsorption-crosslinking onto corn husk. IOP Conference Series: Materials Science and Engineering, 345(1), 012042.
- Bilal, M., et al. (2021). Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. International Journal of Biological Macromolecules,175, 108–122. Google Scholar
- Zucca, P., & Sanjust, E. (2014). Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules,19(9), 14139–14194. Google Scholar
- Datta, S., Christena, L. R., & Rajaram, Y. R. S. (2013). Enzyme immobilization: an overview on techniques and support materials. 3 Biotech,3, 1–9. Google Scholar
- Mateo, C., et al. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology,40(6), 1451–1463. Google Scholar
- Sirisha, V. L., Jain, A., & Jain, A. (2016). Enzyme immobilization: An overview on methods, support material, and applications of immobilized enzymes. Advances in Food and Nutrition Research,79, 179–211. Google Scholar
- Thangaraj, B., & Solomon, P. R. (2019). Immobilization of lipases–A review. Part I: Enzyme immobilization. ChemBioEng Reviews,6(5), 157–166. Google Scholar
- Dong, H., et al. (2013). The study on effective immobilization of lipase on functionalized bentonites and their properties. Journal of Molecular Catalysis B: Enzymatic,95, 9–15. Google Scholar
Acknowledgements
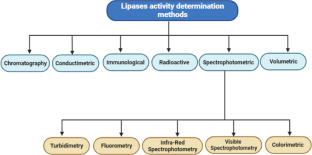
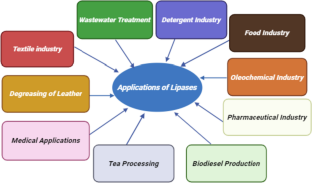
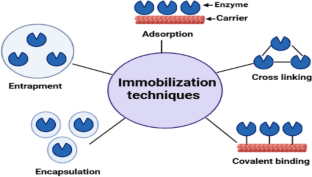
The authors acknowledge the page BioRender.com as the main program to make Figures 2, 3, 4 and 5.








